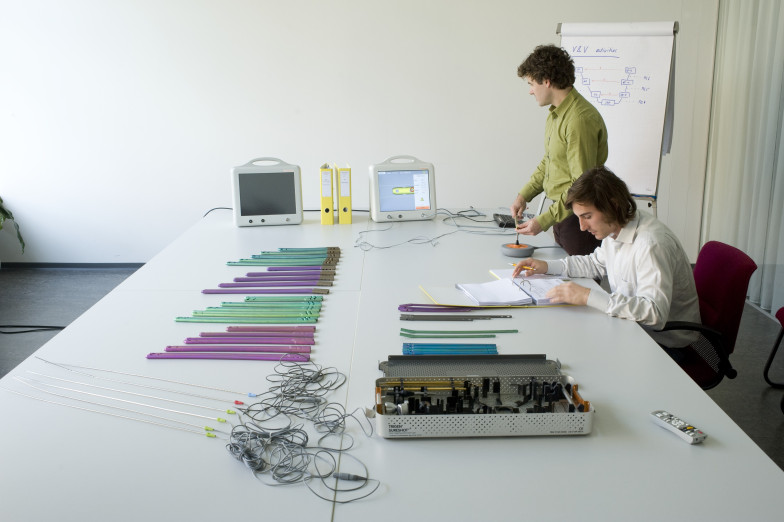
Medivation has extensive experience in the medical regulatory field. We are capable to work either in the customer's development process or develop in our own quality system under a quality plan agreement. Furthermore, we can act as an OEM legal manufacturer for customers who do not want to approve a product on their own.

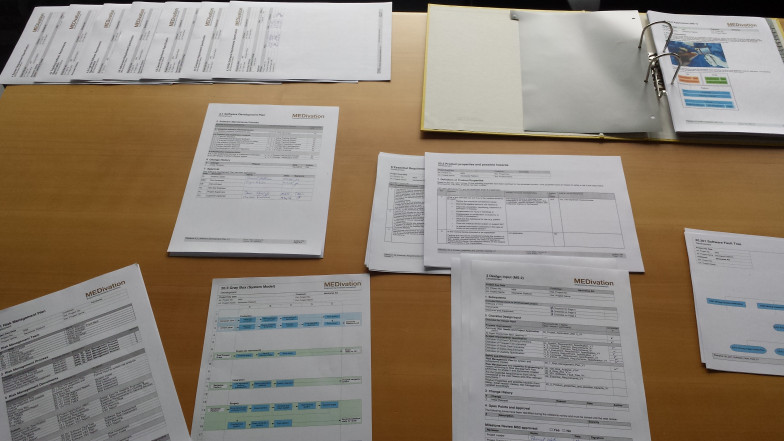
Medivation has certified medical products for CE class I, CE class IIa, FDA class 1 and FDA class 2 products as well as patient specific solutions and has supported customers to obtain regulatory approval in further markets.
- Verification and validation tasks require a deep understanding of the product risks as well as regulatory requirements. Medivation's in-house expertise can fasten the process of the V&V phase and can ensure an independent testing of critical submodules.
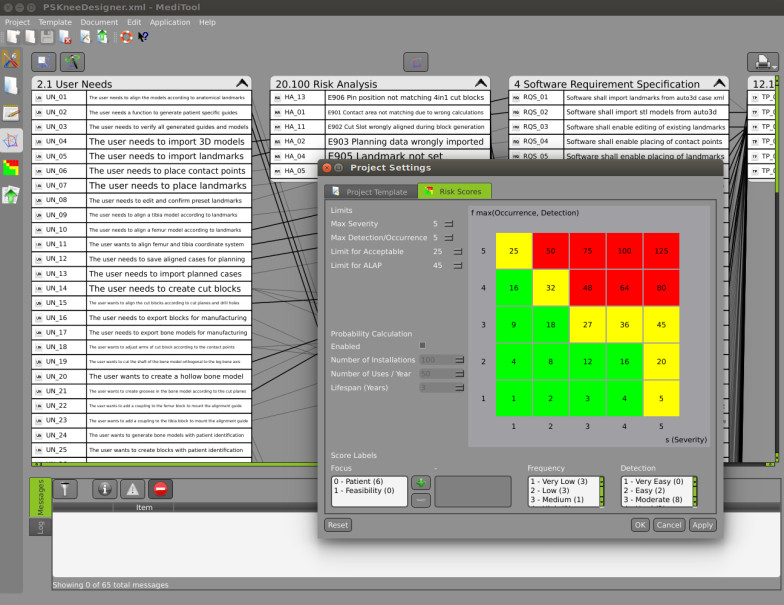
- Our self-developed and validated traceability tool has generated interest throughout the market for being a rather simple and straightforward working tool for traceability and document generation. Please get in contact if you are interested to learn more about the tool.

We manufacture electronic components like control units in small quantities in order to provide customers profound manufacturing documentation. Higher volumes can then be easily transferred to dedicated production companies in the medtech field.
